Whether you like it or not, if you like food, you might have a little bit of a chemist in you! (Trust me, it’s not a bad thing).
While making my way through this week’s reading and class, I was brought back to the days when I watched Top Chef and Iron Chef. What exactly was molecular gastronomy, and who deserved to say they used this technique in their cooking? Now after the class has gone by, I have a pretty good idea, at least of what I think it should be.
While reading the articles posted for this week’s class ( Molecular Gastronomy: A New Emerging Scientific Discipline by Peter Barham et al. and Molecular Gastronomy, a Scientific Look at Cooking by Herve This) I found two different outlooks on molecular gastronomy. One article states that molecular gastronomy is mostly concerned with four properties of food. These are: the physical, chemical, biological, and organoleptic properties of the food. Physical means its state- solid or liquid. Chemical refers to what molecules make up the food—proteins, carbohydrates, or fats. Biological refers to what these molecules do when they get in the body. Organoleptic refers to how we perceive food via our senses—taste, sight, smell and touch.
The second article claims that the goals of molecular gastronomy should be to clarify the conditions under which food becomes “truly enjoyable” and find ways in which to make food so it reaches these conditions. These methods include how we grow our food and which methods we use to cook it once it reaches our kitchen. The paper suggests we systematically categorize the methods commonly used in the kitchen and ask ourselves whether these are good methods.
After completing the experiment with color extraction, which is explained below, our class came up with an even different definition of molecular gastronomy—simply the study of the chemistry (on the micro level) behind food and cooking processes. I believe this definition encompasses both articles’ ideologies within it. The study of the chemistry includes the four principles in the first article, as well as partially explains why some dishes taste so delicious.
Colors in Chemistry
Have you ever bought a substance to put in your campfire in the summer that makes the fire glow different colors? Do you know why this happens? The substances in these packages are usually metals like copper or zinc or magnesium. When heated, the metal’s electrons get “excited” and jump to higher energy levels. When they fall back down, they emit the color of the light spectrum that they absorbed on the way up, which we perceive as various colors. Copper is usually green, for those who have seen this before.
Colors in Food
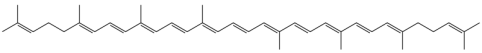
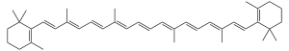
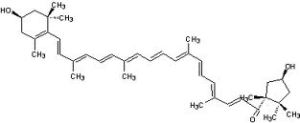
Now, how is this relevant to food? In foods, the colors we observe are from the absence of the colors that the fruits and veggies absorb, which is a little different than copper in the fire. Molecules like beta-carotene (in carrots), and lycopene (in tomatoes) are big long molecules with lots of double bonds:
These double bonds make it easier for electrons to “go upstairs” in a molecule. These bonds cause the foods to absorb certain colors, and we see the true colors, like perceiving tomatoes as red. Tomatoes absorb everything but red. allow the foods to emit color
The colors in these foods and others can be extracted using either water or fat (oil) depending on the chemical makeup of the color-generating compound. The way this is determined is by looking at the structure of the molecule and comparing it to the structure of water or oil. “Like dissolves like” is a term we like to use. If a molecule looks like water—has a charge or has OH groups on it, the color will most likely be extracted by water. On the other hand, if the molecule looks like fat—it will be a long chain with double bonds and no OH groups, the color will most likely be extracted by fat.
In order to test this hypothesis, check out the following experiment. We had an assortment of fruits and veggies. Each picture is a color molecule present in that fruit or vegetable.
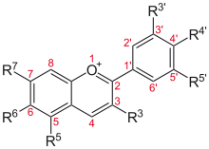
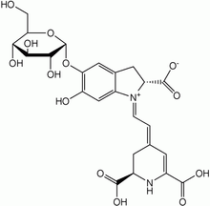
Blueberries, blackberries, raspberries, purple potatoes, artichokes, swiss chard, and cabbage all have some form of anthocyanins:
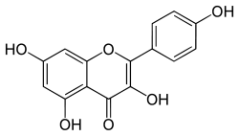
Gold beets and purple beets contain betanin:
Leeks have Kaempferol:
Carrots have Beta-Carotene:
Red peppers have capsanthin:
Red and orange tomatoes have lycopene: 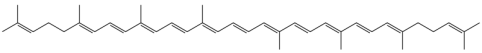
Here is a picture of our “victims” before they were demolished:
We hypothesized that the berries, beets, cabbage, potatoes, and swiss chard colors would be extracted by water rather than fat. This is because we knew that the molecules in some things—like berries, resembled water and would dissolve in it. For the fruits and veggies that had unknown color molecules, (an example of a color molecule is beta-carotene we hypothesized based on observed color of the substances. We hypothesized that the carrot, tomato, and pepper colors would be extracted by fat because their color molecules resembled fats—long chains of hydrocarbons with double bonds (which we call pi bonds).
In order to perform this experiment, we crushed the fruits and vegetables using various tools. We used a blender, stick blender, mortar and pestle, and a food processor for the crunchy and dense things like carrots. Every substance was blended using water except carrots. The potatoes, carrots, and red peppers were blended with oil. Once each substance was pulverized, we strained it into a clear glass for observation.
Pulverized orange tomatoes:
The leeks:
The carrots:
Our hypotheses turned out to be correct. The berry, cabbage, beet, potato, and swiss chard colors were all extracted by water. In addition, the leek and artichoke colors were extracted by water.
When we tried the potatoes in oil, we made somewhat of an emulsion—a mixture in which fats and waters are brought together. The red pepper color and carrot color were extracted using oil. We did not try the tomatoes via oil, but neither tomato color was extracted well by water.
Once each color was extracted, we performed another experiment. We divided the color extracts into 2 portions each and added acid or base. In order to do this, some of the substances were diluted with tap water, with a pH of about 8. This is slightly basic water, typical of a municipal water supply. When the cabbage water was diluted it turned bright blue; a preview of what was to come. Once each color was separated into two glasses, we added base to one (pH of 9.5) and acid to the other (pH 4.5). The pHs were measured using litmus paper—paper that changes color in the presence of an acid or base. The base was a dilute solution of sodium bicarbonate—baking soda—in tap water. The acid we used was vinegar, a 5% acetic solution in water. When adding acid, the raspberry, blueberry, blackberry, beet, artichoke, cabbage, and leek water changed colors. The most striking color change was the cabbage water, which turned bright pink:
When adding base, the same waters changed color except for artichoke. Again the most striking color change was cabbage, which turned bright blue:
This is because cabbage is rich in anthocyanins. These are color molecules that act like a natural litmus paper. In acid they turn red, in base, blue. The fact that the berry waters all reacted with acid and base probably indicates that they have anthocyanins present as part of their color molecules.
As we marched back to the compost heap to dump our concoctions out, I was left pondering the true meaning of molecular gastronomy and how exactly it could be applied to every day life. We decided that it was the study of the micro level of chemistry within food and cooking. In order to study this, sometimes the macro level is involved. I think molecular gastronomy includes how we interact with food on a macro level. This definition certainly counts for chemists, and especially for our class. We performed a macro level experiment in order to understand what was going on in these fruits and vegetables on the micro level. Last week, when we visited the Cornell Agricultural Station, we learned firsthand how chemists study volatiles in foods and drinks—molecules that cause the smells we perceive. When I started taking this class, I wouldn’t have classified myself as a molecular gastronomist. However, now I believe I am. I think about the chemistry and how things work together while I’m cooking. I also try and understand ingredient labels when I purchase things at the store. Turns out you don’t need to be a PhD chemist to understand them! Once you have a background about what some chemicals are (which you can look up online) you can begin to understand how food “works.” This, I think for me, is what makes food truly enjoyable. I like knowing what I am eating and thinking about the micro level. This however, might not be for everyone. Some people might like to just think about what tastes good but not why. This too encompasses molecular gastronomy. This is because taste, smell, texture, and sight are all important facets of molecular gastronomy on the macro level. So whether you like it or not, if you like food, you might have a little bit of a chemist in you! (Trust me, it’s not a bad thing).
So, there’s my two cents on what molecular gastronomy is, and it was pretty fun figuring it out. If you’re interested, try the color extraction experiment with cabbage, or something new that we didn’t try. You might be surprised at what you find out!